MEDTRONIC DRUG-COATED BALLOON SHOWS STRONG CLINICAL AND ECONOMIC BENEFIT IN TREATMENT OF PERIPHERAL ARTERY DISEASE
IN.PACT Global Study Reports Favorable Outcomes in Real-World Patient Population and Validates Key Findings from IN.PACT SFA Trial.
Physicians
and patients are evaluating the available treatment options in light of
this compelling evidence.
At the same time, payers and providers are concerned
with the impact of newer technologies on their budgets.
WASHINGTON Sept. 16, 2014 Presented for the first time at the 2014 Transcatheter Cardiovascular Therapeutics (TCT) conference, the latest clinical and economic data on the IN.PACT Admiral drug-coated balloon (DCB) from Medtronic, Inc. (NYSE: MDT) augments an already robust body of evidence that continues to drive a reconsideration of the standard of care for peripheral artery disease (PAD) in leg arteries above the knee.
The IN.PACT Admiral DCB remains an investigational medical device in the United States. No drug-coated balloon has yet received approval from the U.S. Food and Drug Administration.
The new data come from the real-world IN.PACT Global study, the largest and most rigorous post-market evaluation of its kind for any peripheral artery intervention
Together, the results show that the IN.PACT Admiral DCB is not only clinically superior, but also cost-effective for the treatment of atherosclerotic lesions in the superficial femoral artery (SFA) or proximal popliteal artery (PPA) at 12 months compared to standard percutaneous transluminal angioplasty (PTA).
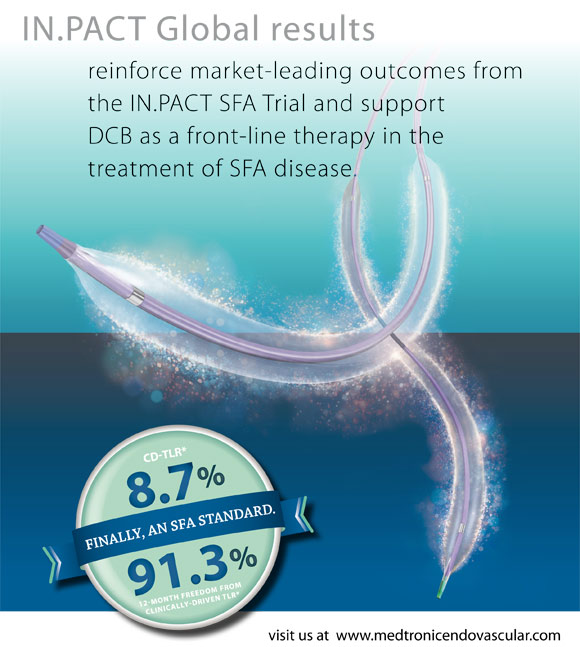
IN.PACT SFA, a randomized controlled trial comparing the IN.PACT Admiral DCB to standard PTA head-to-head, found the lowest 12-month clinically-driven target lesion revascularization (CD-TLR) rate for any peripheral intervention ever studied (2.4 percent vs. 20.6 percent, respectively).
Initial findings from the IN.PACT Global study, which will enroll more than 1,500 patients at 67 sites around the world, characterize the performance of the IN.PACT Admiral DCB in a real-world patient population one with more complex and challenging baseline characteristics than the 331 patients enrolled in the IN.PACT SFA trial. Considering the greater complexity of the patients enrolled in IN.PACT Global, 12-month outcomes for the first 655 patients including a CD-TLR rate of 8.7 percent validate the findings from IN.PACT SFA.
Based on the exceptionally high quality of this real-world, registry-based evidence, the IN.PACT Admiral drug-coated balloon is demonstrating unparalleled outcomes for atherosclerotic lesions in the femoro-popliteal vessel bed, said Gary Ansel, MD, System Medical Director for vascular services at OhioHealth/Riverside Methodist Hospital in Columbus, Ohio. The clinical community of cardiovascular specialists is increasingly excited about the prospects for this interventional technology for the non-prosthetic treatment of peripheral artery disease.
ABOUT MEDTRONIC
Medtronic, Inc. (www.medtronic.com), headquartered in Minneapolis, is the global leader in medical technology alleviating pain, restoring health and extending life for millions of people around the world.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronics periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.